-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Giuseppina Gallucci*, Alba M Capobianco1, Maddalena Maietti, Matilde Gioioso, Loredana Lapadula, Giandomenico Roviello, Leuconoe Grazia Sisti, Nicola Sisti, Aldo Cammarota and Sandro Barni
Corresponding Author: Giuseppina Gallucci, MD, IRCCS CROB Istituto di Ricovero e Cura a Carattere Scientifico. Centro di Riferimento Oncologico della Basilicata, Cardio-Oncology Unit, Rionero in Vulture (PZ), Italy.
Received: April 09, 2023 ; Revised: May 06, 2023 ; Accepted: May 09, 2023 ; Available Online: June 22, 2023
Citation: Gallucci G, Capobianco AM, Maietti M, Gioioso M, Lapadula L, et al. (2023) The Challenging Diagnosis of Pulmonary Embolism in Cancer Patients: Still A Role for D-Dimer Tests? J Can Sci Res Ther, 3(1): 1-4.
Copyrights: ©2023 Gallucci G, Capobianco AM, Maietti M, Gioioso M, Lapadula L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Background: Venous thromboembolism (VTE) is the second leading cause of death in cancer patients. The diagnosis of pulmonary embolism (PE) remains challenging. The D-dimer test (DD) is used to rule out PE, but an elevated value may be misleading in older cancer patients with comorbidities. Age-adjusted values have been suggested, cancer-adjusted values are yet to be found.
Aim: The search of a “tailored” cut-off value of DD to increase the positive predictive value in cancer patients and to avoid overtreatment.
Methods: We retrospectively evaluated the files of 422 cancer outpatients in oncologic treatment: 193 women and 229 men (median age: 67 years): We analyzed their DD values, computed tomography pulmonary angiographies (CTPAs) and cardiology evaluations. DD values were higher than normal (>250 ng/mL) and >500 ng/mL in all patients. We related these DD values to radiological and cardiological evidence of PE.
Results: Among all patients with DD values>500 and CTPAs, we observed 33/422 (7.8%) radiological evidence of PE. Patients with PE had a DD value greater than 800 ng/mL (range: 816-3884).
Conclusions: In our study a higher fixed cut-off value of DD (> 800 ng/mL) performed better than a normal cut-off or an age-adjusted value to diagnose PE in cancer patients. Antithrombotic therapy is strongly recommended in these patients and the issue of prophylactic antithrombotic therapy needs to be urgently addressed considering current therapeutic possibilities.
Keywords: Cancer patients, Pulmonary embolism, D-Dimer values, CTPA
INTRODUCTION
Acute PE is the third most common cause of acute cardiovascular syndrome in Europe after heart attack and stroke. The epidemiology of PE is difficult to determine, and PE is incidentally detected in almost 50% of all patients [1]. Thrombosis is the result of interactions between patient-related and setting-related factors. According to the 2019 ESC Guidelines [2], cancer is a moderate risk factor (odds ratio 2-9) with the highest risk in metastatic disease. In cancer patients VTE is the second leading cause of death [3], and cancer-associated thromboses have a dismal prognosis. Hematological malignancies, lung, gastrointestinal, pancreatic, and brain cancers are associated with the highest risk of VTE. Cancer-related risk is derived from cancer cell secretion of procoagulant factors such as tissue factor (TF)-bearing circulating microparticles [4] and inflammatory cytokines [5]. Surgery and central venous catheters, chemotherapy especially platinum-based therapy, tamoxifen therapy, anti-vascular endothelial growth factor (VEGF) therapies such as bevacizumab, sunitinib, and pazopanib; tyrosine kinase inhibitors, such as nilotinib and ponatinib; immunomodulators such as thalidomide and proteasome inhibitors like carfilzomib increase the risk of venous and arterial thrombosis [6]. Goldhaber called PE “the great masquerader” for its multifaceted clinical features. In the last few decades, clinical probability assessment has become the first strategic step in the diagnostic work-up of PE. The D-dimer (DD) test is currently used as the second diagnostic step. DD levels increase in plasma whenever acute thrombosis is present, due to a simultaneous activation of coagulation and fibrinolysis. The test has a high negative predictive value, but its positive predictive value is very low. Conditions such as cancer, trauma, and surgery can increase the production of fibrin and DD, even without PE. Computed tomography pulmonary angiography (CTPA) is the standard non-invasive imaging modality used to detect pulmonary embolism. In our study, we retrospectively evaluated cancer outpatients with high DD values and CTPA to demonstrate that a higher fixed “cut-off” value of DD would likely increase the specificity of the DD test. At that time, the ESC 2014 Guidelines mentioned the age-adjusted D-dimer values and validated the clinical rules [7].
METHODS
We retrospectively evaluated patients admitted to our institution as outpatients for oncologic treatment and selected those in whom a DD value and a chest CTPA were available. One patient had lung perfusion scintigraphy due to contraindications to CTPA. DD values were quantified using a latex enhanced turbidimetric immunoassay (normal values<250 ng/mL). CTPA was performed using a Toshiba Aquilion 64 (Toshiba Medical Systems, Tokyo, Japan) CT scanner, and images were acquired in the caudocranial direction during a single inspiratory breath-hold and were obtained using a 64x0.75m slice collimation with a tube voltage of 120 kV. The reconstruction slice thickness was 5-1 mm. During CTPA, 100 -120 ml of 370 mg/ml contrast iopromide (Ultravist) was generally administered intravenously at a rate of 2.5- 3 ml/s with a power injector followed by 30 ml normal saline. Lung perfusion scan was performed using albumin macroaggregates. The study protocol was approved by the local institutional ethics board.
RESULTS
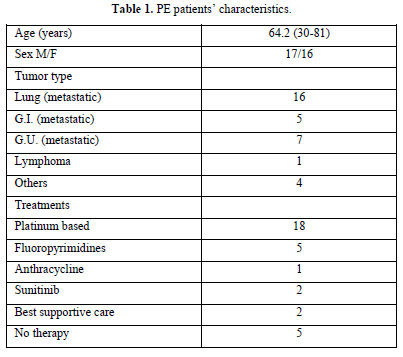
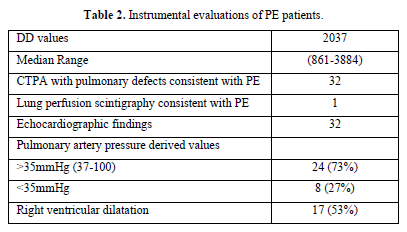
We analyzed the files of 422 patients (193 women, 229 men; mean age, 67 years). All 422 patients had a DD value higher than 500 ng/mL, and 33/422 (7.8%) PE events were detected: CTPA evidence of filling defects in 32 cases and high probability of lung perfusion scintigraphy in 1 case. We found a DD value greater than 800 ng/mL (range 816-3884) in all 33 patients with PE: 1 patient had lymphoma, 32 patients had metastatic disease (Table 1). Among patients with PE, 73% showed abnormal pulmonary artery pressure (PAP) values (PAP greater than 35 mmHg) estimated from echocardiographic Doppler study (range 37-100 mmHg) and 53% echocardiographic right ventricular dilatation (Table 2). None of the patients had a “high risk” clinical presentation of shock or hypotension at admission, all patients had a diagnostic work-up before CT.
DISCUSSION AND PERSPECTIVES
The diagnosis of PE remains challenging. In 1990 the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study determined the probability of PE by clinicians’ global judgement [8], but the validity of an empirical judgement “Gestalt” [9] was subsequently questioned and in the following years the scores were mostly based on clinical criteria: Wells score [10] and Geneva score. In 2006, LeGal and Perrier proposed a revised Geneva score made of “objective” clinical features [11]. According to the 2019 Guidelines “regardless of the score used, the proportion of patients with confirmed PE is expected around 10% in the low-probability category, 30% on the moderate-probability category and 65% in the high-probability category” [2]. PE rule-out criteria (PERC) have been proposed in the emergency department to select patients with a low likelihood of PE, in which no diagnostic work-up is deemed necessary. The eight clinical variables associated with a low likelihood of PE were age<50 years, heart rate<100 beats per minute, SaO2 >94%, no unilateral leg swelling, no hemoptysis, no recent trauma or surgery, no history of VTE, and no hormonal use [12].
In cancer patient’s empirical judgement (“Gestalt”) and clinical assessment with a “subjective” issue (“alternative diagnosis less likely than PE”) may have a more powerful diagnostic yield. On the contrary, the value of D-Dimer has been questioned. The specificity of DD in excluding PE decreases with age; age-adjusted D-Dimer cut off levels calculated as “age x 10” in patients 50 years or older, have been tested by Righini [13] in a multicenter, multinational prospective outcome study with a population of 3346 patients; pretest clinical probability coupled with age-adjusted DD cutoffs could rule out PE with a failure rate of 0.3%. [13]. A recent study tested the positive predictive value of DD in a general population of 370 patients. Very high DD values, around 2000 or at least four times the normal value, increased the likelihood of PE even in patients with a low clinical probability, mandating a CTPA, suggesting that higher numbers (>2000) should be applied in cancer patients [14]. D-Dimer values were linked to clinical pre-test probability to rule out PE in a prospective study of 2000 patients by Kearon [15] Pulmonary embolism was ruled out without other testing in outpatients with a low clinical pre-test probability and a DD value less than 1000 ng/ml or with a moderate clinical pre-test probability and a DD value less than 500 ng/ml. These patients showed a low risk of PE during a 3-month-follow up with no anticoagulant therapy [15].
In very elderly patients with suspected PE in the emergency department, a fixed higher DD value (1000 ng/mL) increases the test specificity for PE without reducing its sensitivity, as shown by Friz [16].
In patients with cancer, a higher fixed DD cut-off value may increase the specificity. In our oncologic population a higher fixed “cut off” value of 800 performed better than age-adjusted DD values to diagnose PE, but the high number (almost four times the normal value) suggested by Sikora-Skrabaka [14] may have an even better predictive value and should be tested in a prospective study. In all cases an integration of the DD value with clinical pre-test probability is necessary in the diagnostic workup of PE.
As a matter of fact, the aim of all these rules is to avoid unnecessary CTPAs in a general population of patients, in which an increased awareness of over treatment has been emerging in these last years. On the contrary, in cancer patients, a high percentage of incidental findings and a possible under treatment may be a relevant issue. Moreover, a 3% false-negative result of D-Dimers was described in a retrospectively reviewed population of 8023 cancer patients [17]. With these caveats in mind, we think that the work-up of PE diagnosis in cancer patients should not end with an answer to the question: is this a case of PE? What we really need to answer is a more intriguing question; does this patient require a safe and personalized anticoagulation? The answer is yes in most of the cases: the “ruling in” criteria is more important than the “ruling out”. In metastatic cancer patients, anticoagulation is frequently indicated, the only limitation being hemorrhagic risk. We should also consider the prognostic value of episodes of VTE and of high pretreatment DD levels independently associated with poor overall survival.
Moreover, the issue of thromboprophylaxis has to be carefully addressed in cancer patients during oncologic treatment. Besides the Khorana score, the type of tumor, the stage, the oncological therapy [18] and the site of the tumor must be considered.
In addition, the possible link between venous and arterial thrombi should not be neglected, the shared risk factors between arterial and venous thrombosis point to a common underlying abnormality whose main player is a dysfunctional endothelium [19].
In recent years the interplay between immunity and thrombogenicity, the hallmark of the intriguing thrombotic phenotype of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic, has fueled many studies to decipher molecular networks of thrombosis and emerging markers of endothelial dysfunction and thrombosis [20]. It is possible that in the near future extracellular vesicles will become valuable diagnostic and therapeutic tools in many conditions of inflammation and inflammageing [21] and neutrophil extracellular traps may represent therapeutic targets of inhibitors that will not interfere with the hemostatic pathway [22].
CONCLUSIONS
PE is still a challenge, especially in cancer patients. Clinical judgment and tailored values for DDimers may help in the diagnostic process; in our observational study we found that a fixed higher number may increase the predictive values of DD in the workup of PE, but it would be interesting to validate the higher numbers suggested by Sikora-Skrabaka [14]. Increased “tailored” DD values can help in the management of cancer-associated thrombosis.
LIMITS
The main limits of our study are represented by the small sample of patients and the retrospective evaluation of data. A larger number of patients and a prospective study coupled with an analysis of new markers of thrombosis in the “thrombophilic” milieu of cancer patients will likely increase our ability to predict VTE and PE.
No Files Found
Share Your Publication :