-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Rafiye Ciftciler*, Ali Erdinc Ciftciler, Adnan Batman and Mustafa Ekici
Corresponding Author: Rafiye Ciftciler, Associate Professor, Department of Hematology, Aksaray University, Training and Research Hospital, Aksaray, Turkey
Received: April 14, 2022 ; Revised: May 09, 2022 ; Accepted: May 12, 2022
Citation: Ciftciler R, Ciftciler AE, Batman A & Ekici M. (2022) Evaluation the Effect of Laboratory Parameters and Comorbidities of COVID 19 Patients at the Time of Diagnosis the Mortality and Length of Hospital Stay. J Can Sci Res Ther, 1(2): 1-7.
Copyrights: ©2022 Ciftciler R, Ciftciler AE, Batman A & Ekici M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
Background and Aim: The novel coronavirus (SARS-CoV-2) that causes novel coronavirus pneumonia (COVID-19) is the third fatal coronavirus globally. The prognosis of disease varies depending on patient’s specialties. In this study, our aim was to evaluate whether hematological, biochemical parameters and patients’ comorbidities predicted the mortality of patients and whether they had an effect on the duration of hospitalization in patients treated by hospitalization.
Materials and Methods: In this retrospective study, 1132 patients hospitalized at our care center between July 2020 and December 2020 was evaluated.
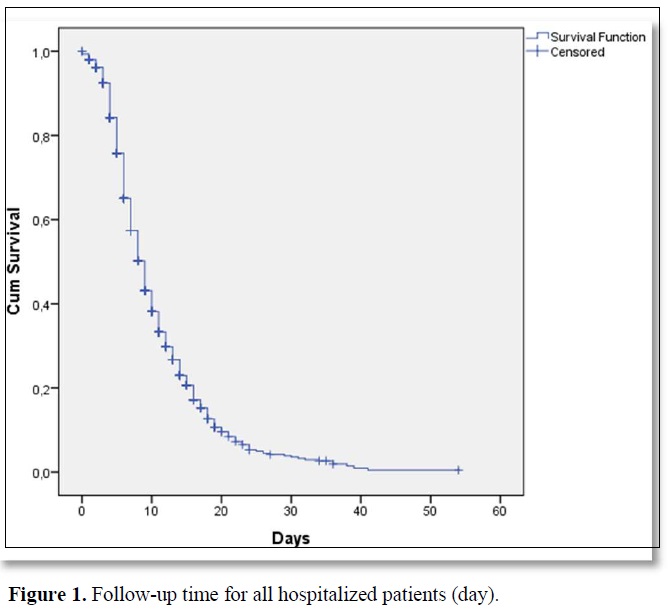
Results: A total of 1132 patients who were hospitalized between July 2020 and December 2020 were included in the study. All of the patients were hospitalized with COVID 19. There were 542 (47.9%) males and 590 (52.1%) females with a median age of 71 (19-98) at the time of hospitalization. The median follow-up time was 8 (range, 0-54) days in hospital for all patients. Mortality was observed in 237 (20.9%) of all these patients who were hospitalized and followed up.
Conclusion: In conclusion, this study indicates that by using the most simple and routine laboratory parameters at the time of COVID-19 diagnosis, it may be possible to predict a patient's prognosis. This will help patients receive early clinical care, reducing patient mortality and aiding in the control and prevention of the outbreak.
Keywords: COVID-19, Novel coronavirus, Complete blood counts, Ferritin, Prognosis
INTRODUCTION
The novel coronavirus (SARS-CoV-2) that causes novel coronavirus pneumonia (COVID-19) is the third fatal coronavirus all over the world. It first emerged in December 2019 in China [1]. The most common symptoms are fever, dry cough, and exhaustion. Dyspnea and/or hypoxemia are common one week after symptoms in severe patients. Acute respiratory distress syndrome (ARDS), septic shock, difficult-to-correct metabolic acidosis, coagulation deficiency, and multiple organ failure can occur rapidly in severe cases [2,3]. Patients with mild clinical manifestations do not require hospitalization at first, however they may develop respiratory symptoms by the second week, so all patients should be closely monitored [4]. According to the World Health Organization (WHO), approximately 80% of sick people have mild to moderate infections (including those with or without pneumonia), 13.8% have serious infections, and 6.1% have a critical illness [4]. The prognosis of the disease varies depending on patient specialties. As a result, identifying and diagnosing severe or critical patients is essential. Laboratory analysis is the most commonly conducted procedure in clinics, and complete blood count (CBC) and biochemical findings are the first things that physicians want to see in nearly all labs, outpatient and inpatient clinics. In the current novel coronavirus pandemic, it would be beneficial for clinicians to make a rational allocation of medical resources if the most routine and affordable laboratory tests can be used to provide clinicians with convenient assistance in assessing the patient's condition. Early clinical intervention is expected to minimize patient mortality [4]. It has been shown that the total number of peripheral white blood cells is normal or the number of lymphocytes is decreased in patients in the early stage of COVID-19. Tan et al. reported that lymphocyte percent was inversely linked to patient severity and prognosis, and could be used to predict COVID-19 patient’s severity and prognosis [5]. This means that patients with SARS-CoV-2 infections will experience changes in their peripheral blood. These adjustments can hold clues or provide guidance for COVID-19 patients' diagnosis, care, and prognosis.
The aim of this study was to correlate hematological, biochemical parameters and patients’ comorbidities at the time of diagnosis with the prognosis of patients hospitalized due to COVID-19.
MATERIALS AND METHODS
Patients
In this retrospective study, 1132 patients hospitalized at our care center between July 2020 and December 2020 was evaluated. Recommended criteria established by the Scientific Committee of the Ministry of Health were used in the selection of definite COVID-19 patients [6]. The diagnosis of COVID-19 was made according to the guidelines of the Ministry of Health of Turkey and confirmed by real time polymerase chain reactions (RT-PCR) performed on respiratory samples of the patient [6]. All of the cases included in the study were patients with moderate and severe symptoms and need hospitalization. Patients who had acute respiratory tract infection in the last 14 days were hospitalized and followed up in case of severe infection, fever, cough, dyspnea, tachypnea, hypoxemia, hypotension, diffuse radiological findings on lung imaging or change in consciousness [6]. Patients who had missing laboratory results, transfers to other medical facilities with uncertain outcomes, and patients under 18 years of age were excluded from the study.
Data collection
The date of admission, demographic data of the patients, clinical status and laboratory findings at the time of admission, and clinical course of the patients were obtained from the patient files and hospital database. As a result of application standards of the hospitals, it has been recognized from the patient records that all of the studied patients had given informed consents at the time of hospitalization and before the administration of relevant diagnostic/therapeutic standards of care. Patients gave informed consent for the procedure. This study was approved by the Committee on the Ethics of Aksaray University Faculty of Medicine (2021/04-10).
Statistical analyses
Statistical analyses were performed using the SPSS software version 25. The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorow-Simirnov/Shapiro-Wilk’s test) to determine whether they are normally distributed or not. Descriptive analyses were presented using means and standard deviations for normally distributed variables. Comparisons were made using the t test, chi-square test, Fisher’s exact test, and analysis of variance. Variables that are found to be significant (p < 0.05) in univariate analysis were tested in multivariate analysis, which was performed using a stepwise logistic regression model. Values of p < 0.05 were considered statistically significant. Survival analyses were made using Kaplan-Meier test.
RESULTS
General Characteristics of hospitalized COVID-19 Patients
A total of 1132 patients who were hospitalized between July 2020 and December 2020 were included in the study. All of the patients were hospitalized with COVID 19. There were 542 (47.9%) males and 590 (52.1%) females with a median age of 71 (19-98) at the time of hospitalization. The median follow-up time was 8 (range, 0-54) days in the hospital for all patients (Figure 1). 897 patients (79.2%) were followed-up in COVID 19 clinics. These patients did not need intensive care in their hospitalization and follow-up. It was observed that 235 patients (20.8%) needed intensive care in the diagnosis or follow-up. Five hundred eighty-six (95.4%) of the patients who did not need intensive care were discharged during follow-up. Forty-one (4.6%) of the patients who did not need intensive care died in the service. Thirty-nine (16.6%) of the patients who needed intensive care were discharged during follow-up. One hundred and ninety-six (83.4%) of the patients who needed intensive care died during follow-up. A statistically significant difference was observed between the mortality rates of patients who needed and did not need intensive care (p<0.001). The median time for admission intensive care unit was 3 (0-21) days for patients who needed intensive care follow-up. Sixty-seven of these patients (5.7%) needed an intensive care unit on the day which they admitted to the hospital. Mortality was observed in 237 (20.9%) of all these patients who were hospitalized and followed up.
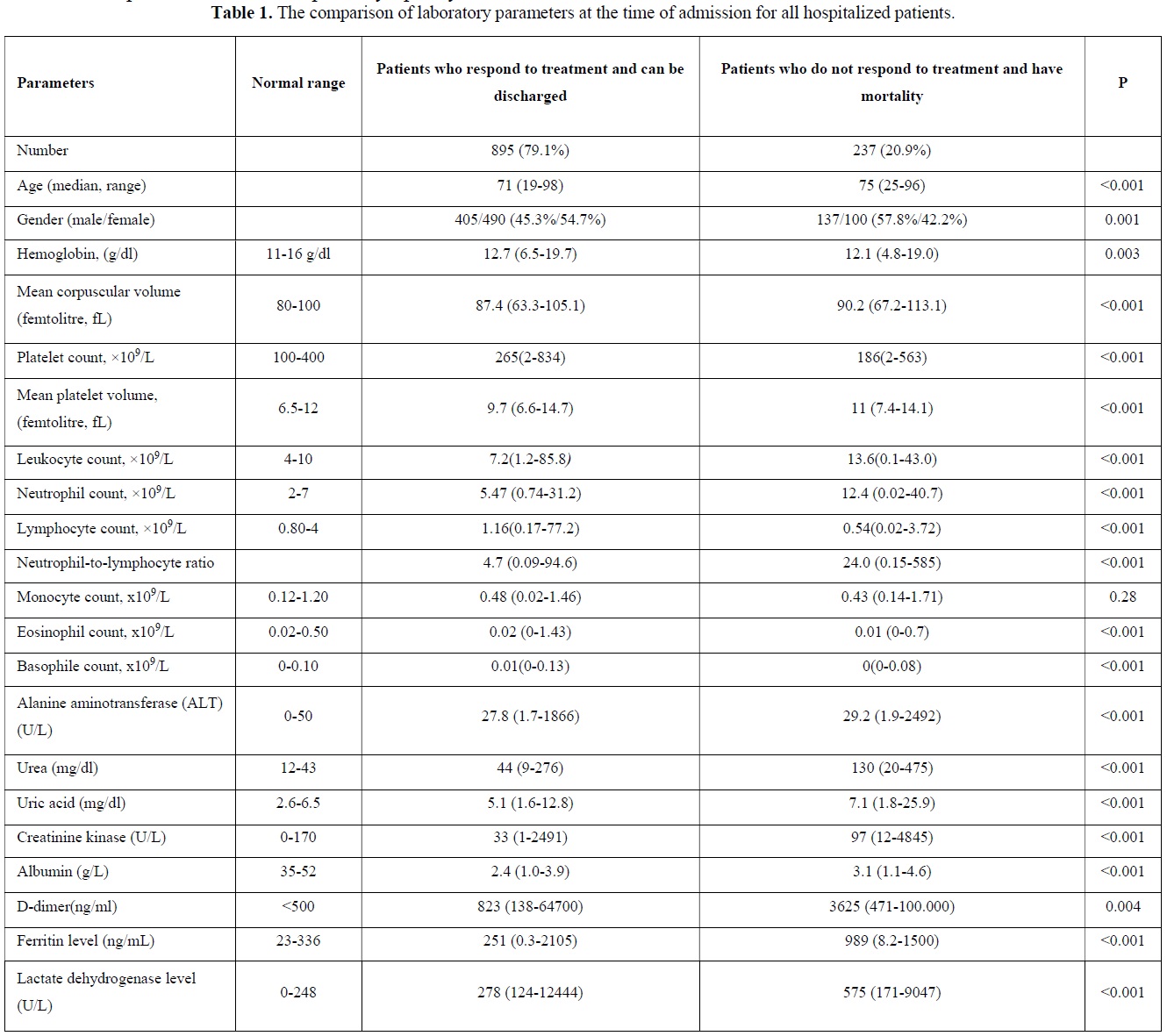
Laboratory findings of COVID-19 patients at the time of admission
The comparison of laboratory parameters at the time of admission of patients who respond to treatment and can be discharged and patients who do not respond to treatment and have mortality were depicted in Table 1. Median age was significantly higher in patients who do not respond to treatment and have mortality (p<0.001). Most of the patients with mortality were males (p=0.001). There was a statistically significant difference in the parameters other than monocyte among the hemogram parameters checked at the time of presentation. Neutrophil / lymphocyte ratio was significantly higher in patients with mortality (p<0.001). Additionally, when we evaluated ferritin (p<0.001), D-dimer (p=0.004) and lactate dehydrogenase (LDH) (p<0.001), among other parameters, mortality was found to be significantly higher in both parameters at the time of admission to hospital. Among the other biochemical parameters of the patients, alanine aminotransferase (ALT), creatine kinase (CK), urea and uric acid were found to be statistically significantly higher (p<0.001) at the time of admission to the hospital in patients with mortality, while albumin was found to be statistically significantly lower (p<0.001).
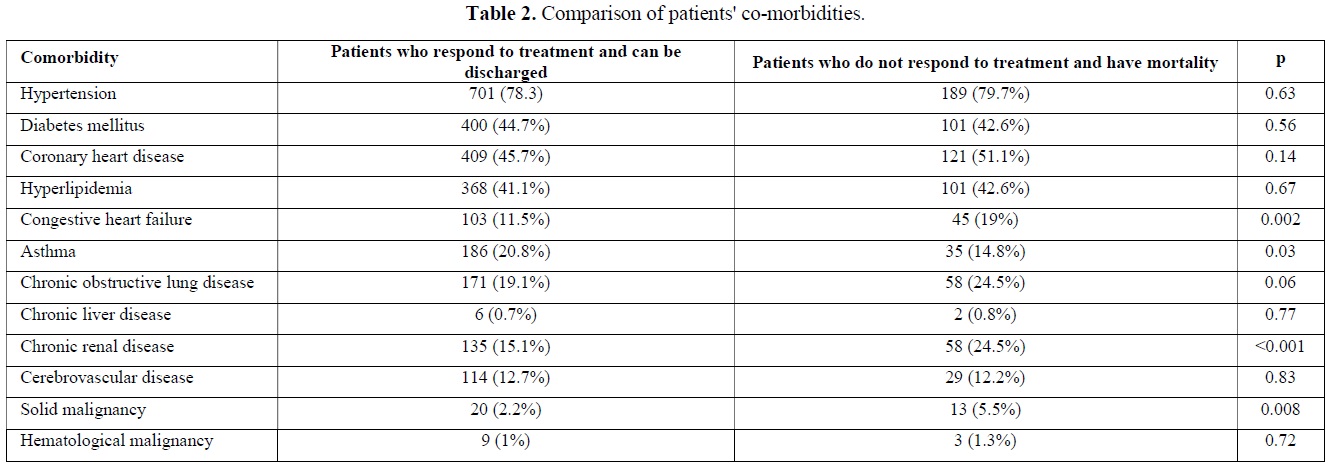
The Comparison of COVID 19 patients' co-morbidities
Co-morbidities of patients who required hospitalization due to COVID 19 were evaluated. When the comorbidities of the patients who died and were discharged during the follow-up were compared, a statistically significant difference was observed between the two groups in terms of having congestive heart failure (p=0.002), asthma (p=0.03), chronic renal disease (p<0.001) and solid malignancy (p=0.008) (Table 2).
After univariate analysis, among the factors that had p value
Clinical Course of COVID-19 Patients according to laboratory findings
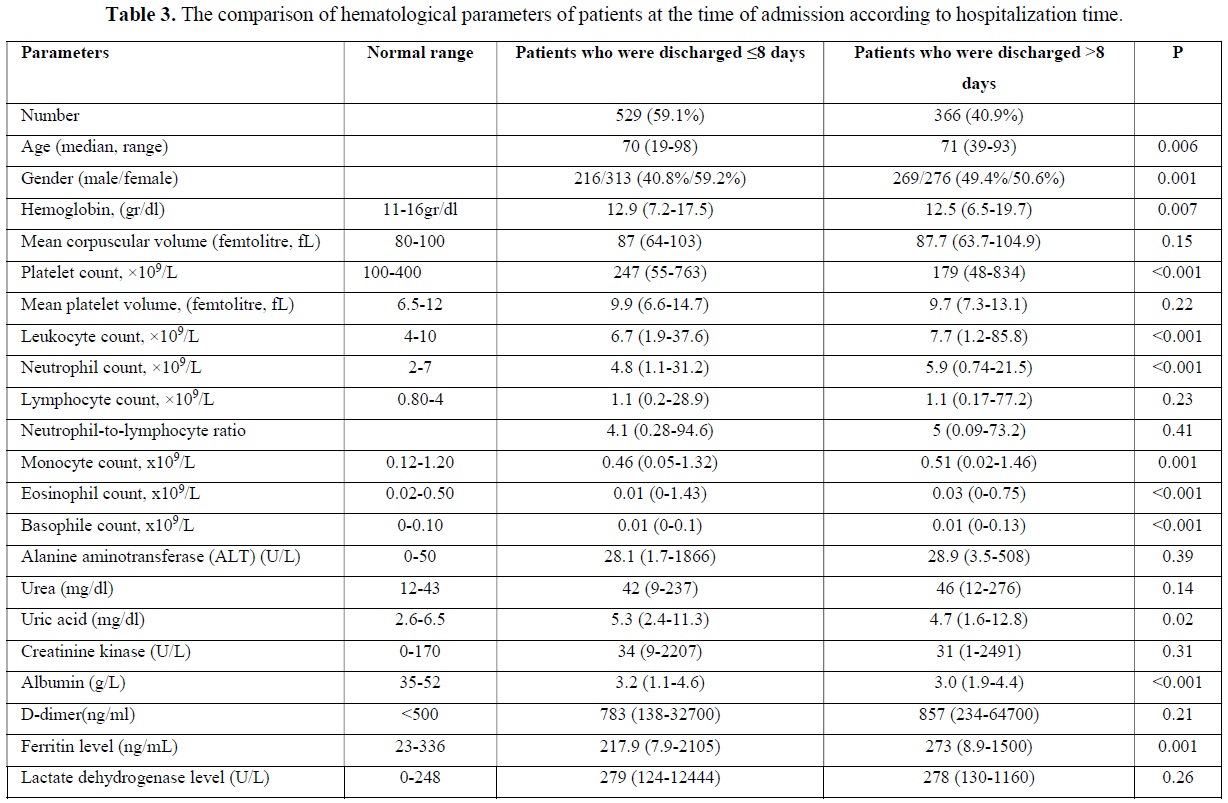
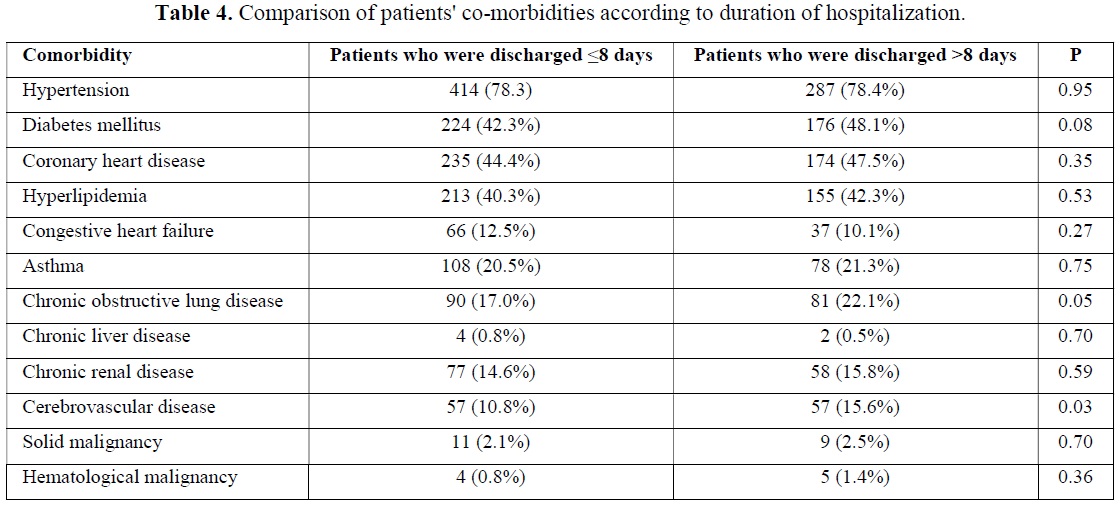
A total of 895 (79.1%) patients recovered during follow-up and were discharged. Median follow-up time was 8 (0-54) days for those patients in the hospital. Five hundred and twenty-nine (59.1%) of these patients were discharged in 8 or fewer days. Three hundred and sixty-six patients (40.9%) were followed up in the hospital for more than eight days and were discharged after a maximum of 54 days. The median age was statistically significantly higher in patients who stayed longer in the hospital (p=0.006). The majority of the patients who were hospitalized for shorter periods consisted of women, while the proportions of both sexes were almost equal in longer hospitalization periods. The hemoglobin and thrombocyte level at the time of admission were statistically significantly higher in patients who were discharged in ≤8 days than in patients who were discharged in >8 days. At the time of admission, the leukocyte, neutrophil, monocyte, eosinophile, basophile and ferritin values were statistically significantly lower in patients who were discharged in ≤8 days than in patients who were discharged in >8 days (Table 3). When we evaluated the comorbidities between the two groups, a statistically significant difference was observed between the patients who had the only cerebrovascular disease in terms of length of hospital stay (p=0.03) (Table 4).
DISCUSSION
In the last two years, the prevalence of COVID-19 infection has increased daily among all over the world. As a result, determining the severity and mortality of the disease is necessary in order to minimize the pandemic's spread. Many studies have identified clinical features of COVID-19 patients, including epidemiological, clinical, laboratory, radiological, and treatment results. Laboratory findings at the time of hospital admission were shown as important differences between severe and non-severe patients. In addition, some previous studies have revealed the role of several laboratory parameters in evaluating the disease severity of COVID-19 in hospitalization [3,7-9]. In this study, our aim was to evaluate whether hematological, biochemical parameters and patients’ comorbidities predicted the mortality of patients and whether they effected on the duration of hospitalization in patients treated by hospitalization.
It has been stated that as the age of infected patients rises, so does the mortality rate, with the crude mortality rate in people over 80 years old being 21.9% [4]. Multivariate analysis showed that old age and high concentration of LDH were independent predictors of poor prognosis in a retrospective study [10]. As a consequence, identifying and diagnosing severe or critical patients is vital. This study showed that the patients' median age was 71 years, ranging 19 to 98 years. In a study comparing moderate and severe patient groups, no significant difference was found in terms of gender [4]. It was observed that the number of female patients hospitalized was higher than the number of male patients in this study. However, most patients who did not respond to treatment and had mortality were males. Female gender was observed more frequently in patients who responded to treatment and were discharged. In addition, it was observed that the length of hospital stay was shorter in women.
According to a meta-analysis report, COVID-19 severity is associated with higher WBC counts and lower lymphocyte, CD4+ T cell, and CD8+ T cell counts in COVID-19 patients [11]. The decrease in lymphocyte count appears to be directly proportional to the severity of COVID-19 infection. Wang et al. reported that leukocyte, neutrophil, NLR, platelet-to-lymphocyte ratio in the severe group were significantly higher than those in the moderate group; additionally the study reported that lymphocyte, eosinophile, and hemoglobin in the severe group were significantly lower than those in the moderate group [4]. In our study, leukocyte, neutrophil and NLR levels were found to be statistically significantly higher in patients with mortality. At the same time, hemoglobin, lymphocyte, eosinophil and albumin levels were found to be statistically significantly lower in patients with mortality. This may be due to long-term infection and hypoxia, which allows the bone marrow to compensate by producing more granulocytes. SARS-CoV-2 can continue to invade more lymphocytes, proliferate, and cause lymphocytes to die or become exhausted when they enter the spleen and other immune organs, resulting in severe lymphopenia in patients with mortality [4]. Fan [12] reported that mild thrombocytopenia and leukopenia were found in some COVID-19 positive patients at first admission. Additionally, they showed that on admission; older age, lymphopenia and raised LDH were associated with intensive care unit admissions [12]. In previous years Xu et al. reported that thrombocyte counts are substantially low in pneumonia patients, and this drop is directly proportional to the patients' clinical status [13].
In many studies conducted across the world, the presence of comorbidities has been shown to be a risk factor for death in patients with COVID-19 pneumonia [16-18]. One study reported that nearly 61% of patients had comorbidities, according to their findings. The most frequent conditions were hypertension (41.25%) and diabetes (41%). Approximately 66.5% of patients with comorbidities died as a result of their disease [19]. In our study, asthma, congestive heart failure, chronic kidney disease and solid organ malignancy were observed statistically significantly more frequently in the mortality group. However, the contribution of these comorbidities to mortality could not be demonstrated in the multivariate analysis. Borderline statistical significance was observed only in patients with solid organ malignancy. Additionally, between the two groups, a statistically significant difference was observed between the patients who had the only cerebrovascular disease in terms of length of hospital stay.
Our study had some limitations. First, this study was retrospective. Second, all patients included in the study consisted of hospitalized patients due to their symptoms. Outpatients with mild symptoms were not included in the study and blood values were not known at the time of diagnosis. However, in this study, it has seen that it is important to associate the onset of symptoms (days of illness) with hematological and biochemical parameters. In conclusion, multiple laboratory parameters can be related to the severity and mortality of COVID-19 infection and should be screened and assessed on a regular basis as the pandemic progresses. WBC count, neutrophil, lymphocytes, NLR, eosinophil, platelet count, ferritin, ALT, urea, uric acid, CK, albumin, D-dimer, and LDH were among the parameters tested. This study indicates that by using the most simple and routine laboratory tests at the time of COVID-19 diagnosis may be possible to predict a patient's prognosis. This will help patients receive early clinical care, reduce patient mortality and aid in the control and prevention of the outbreak.
CONFLICT OF INTEREST
The authors of this paper have no conflict of interests, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included.
ROLE OF THE FUNDING SOURCE
None.
INFORMED CONSENT STATEMENT
Informed consent for the procedure was obtained from blood donors for this study.
REFERENCES
No Files Found
Share Your Publication :