-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Ali Ali*, Wissam Zam and Walaa Ibrahim
Corresponding Author: Ali Ali, Department of Food Technology, Faculty of Technical Engineering, Tartous University, Tartous, Syria
Received: May 15, 2022 ; Revised: July 05, 2022 ; Accepted: July 08, 2022 ; Available Online: Sep 08, 2022
Citation: Ali A, Zam W & Ibrahim W. (2022) Formulation and Evaluation of Chewable Lozenges Containing Myrtle Berries, Cinnamon and Cloves for Oral Disinfection. J Stem Cell Ther Res, 1(1): 1-8.
Copyrights: ©2022 Ali A, Zam W & Ibrahim W. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
Background: Oral diseases are among the major health problems and are listed among the most common of the chronic diseases. Therefore, the attention to oral health is necessary for general health.
Objective: The primary objective of this study was the development of chewable lozenges containing some natural extracts and the evaluation of their physiochemical properties with inspection of their local beneficial effect for oral health.
Method: An ethanolic extract containing myrtle berries, cinnamon and cloves was prepared by maceration for 60 days and used for the formulation of chewable lozenges batches by molding technology using gelatin, salep and pectin as gelling agents.
Results: Results showed that batches containing 1.5 g of gelatin and 0.2 g of salep either with or without sugar have the best physicochemical properties, highest polyphenols release rate at both saliva and gastric simulated media, and were stable for three month at storage temperature (4ºC). The chewable lozenges also showed a diameter of inhibition zone of 14 mm against Streptococcus pyogenes, and 17 mm against Streptococcus pneumonia.
Conclusion: It could be concluded that the prepared chewable lozenges could be a good herbal alternative medication for the disinfection of mouth and the maintaining of the general health.
Keywords: Natural extracts, Chewable lozenges, Physicochemical properties, Herbal alternatives, Oral health
INTRODUCTION
Lozenges are various-shaped, oral forms usually containing a flavoring substance and a medicinal agent [1]. They have become very widespread nowadays, both in food and pharmaceutical preparations, as a means of delivering different active substances [2]. They are premeditated to reduce both local and systemic symptoms as the medical agents are well absorbed via the buccal lining or when swallowed [3]. Lozenges provide various advantages as they are easy to administer to both pediatric and geriatric population and easy to prepare, with minimum amount of equipment and time [2]. Chewable lozenges are one of the most popular lozenges prepared by molding gelatin base which gives a distinctive rubber texture ranging from mild to very solid, depending on the amount of gelatin used [4,5]. Sugar is often added during the preparation of chewable lozenges for the flavor and consistency, but due to its undesirable effects, in terms of increasing glycemic index and high calories from simple sugars, some products are sugar-little or sugar-free [6].
The oral cavity is the main entry for both the gastrointestinal and respiratory systems. Therefore, poor oral health could greatly impact many chronic conditions and systemic diseases [7]. Microorganisms found in the human oral cavity, referred to as the oral microflora [8], count for over than 700 bacterial species [9]. Streptococcus sp. is the most common oral microflora that plays an important role in the human microbial community and human health. Therefore, imbalance of microbial flora, known as dysbiosis, is linked to oral inflammation and could contribute to oral diseases and to systemic conditions through bacteremia [9-11]. A large number of synthetic drugs are currently considered to be effective for the remission of oral and throat inflammation and infection [12]. However, the development of resistant bacterial strains of antimicrobial drugs, the associated side effects of these synthetic medicines, such as vomiting, diarrhea and tooth staining, and possibility of altering oral microbiota by these synthetic medicines have resulted in diversion of research toward screening of natural products [13,14]. So, the blind dependence on synthetics is over and people are returning to natural alternatives with the hope of safety and security. Herbal medicine, also called phytochemistry, has been used for the treatment of several diseases in the oral cavity [14] considered to be safe, effective, more acceptable and compatible with the human body [5].
Myrtus communis L. (common myrtle) is an aromatic evergreen shrub, from the Myrtacea family, growing wild all around the Mediterranean basin, and has important essential oils, tannins and anthocyanin. It considers of the most important drugs being used in Unani system of medicine [15]. Several studies have confirmed the antimicrobial properties of myrtle extracts and essential oils [16,17].
Eugenia caryophyllata (cloves) are the dried, unopened inflorescence (buds) of the clove tree, which belongs to the Myrtaceae family [18]. It have been reported that clove buds extracts and their essential oils have various biological activities such as antimicrobial [19,20].
Cinnamomum verum (cinnamon) is a spice obtained from the inner bark of several trees from the genus Cinnamomum, which belongs to the Lauraceae family. It is used in sweet and flavored savoury foods [21]. Cinnamon has number of medicinal uses and significant biological properties due the presence of active components such as Cinnamaldehyde, Eugenol, and Cinnamic acid [22]. It had been found that cinnamon essential oil have antimicrobial activity against C. perfringens [23]. Another study has been showed that the MIC of cinnamon bark extract against P. acne was 256 µg/mL, while that against S. epidermidis were 1024 µg/mL [24].
The myrtle berries, cinnamon and cloves ethanolic extract is a popular beverage containing large amounts of polyphenols with a high antioxidant activity [25]. This extract was previously tested for its antimicrobial activity and results showed that the extract is mostly effective against S. aureus and L. monocytogenes at 50 mg/mL and 80 mg/mL, with the highest inhibition zones 22 mm and 17 mm [26]. Many other studies also provide strong evidence that these compounds possess a high antimicrobial activity helping preventing many diseases [27,28], including oral diseases such as dental caries and periodontal diseases [29,30].
Therefore, the main objective of the current study was to prepare and evaluate the physicochemical properties of different formulations of chewable lozenges, containing evaporated extract of myrtle berries, cinnamon and cloves either with or without sugar. Their antibacterial effect against Streptococcus pyogenes and Streptococcus pneumonia was also tested in order to investigate their effective oral antiseptic properties.
MATERIALS AND METHODS
Preparation of the extract
The myrtle blue dark color berries were collected from a mountainous region of Safita (in the Syrian coast) in December 2018. Cinnamon bark and clove buds samples were bought from local markets and then ground in a grinder (Type: Moulinex, France).
Cinnamon, cloves and myrtle berries were mixed in a ratio of 1:1:10 (w/w) and soaked with ethanol (50 %, locally produced from grape wine via distillation) at a ratio of 1:10 (w/v). Then the jars containing the macerates were covered by foil and stored in the dark at room temperature for two months. After that, the extract was filtered and the solvent was evaporated using a rotary evaporator (BÜCHI Labortechnik AG, Switzerland) at 40°C, until the solvent in the mix was completely removed.
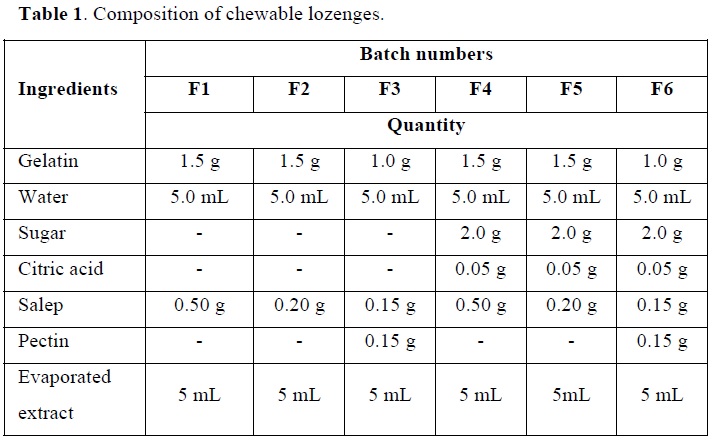
Preparation of chewable lozenges
Six different formulations of chewable lozenges were prepared as indicated in Table 1. These formulations were prepared using gelatin, salep and pectin (HiMedia, India) as gelling agents at different concentrations. Three of them contained sugar and the others were without sugar. Citric acid (HiMedia, India) was used with sugar-containing formulations to prevent the crystallization of sucrose and maintain an appropriate pH lower than 7.
Accurately weighed quantities of gelatin, salep and pectin were mixed in a beaker and added to the required amount of water (with or without the addition of sugar depending on the formulation). The mixture was put in a water bath with constant stirring until the ingredients homogenize. Finally, the evaporated extract was added with good stirring. The final blend was poured into molds and put in the fridge at 4°C.
Evaluation of prepared chewable lozenges
Appearance
The prepared chewable lozenges were inspected visually for color, clarity and the presence of any suspected particles [5,31].
Stickiness and grittiness
Texture of the prepared chewable lozenges in terms of stickiness and grittiness had been evaluated by visual inspection of the product after mildly rubbing the lozenge sample between two fingers [5,31].
pH
The pH of all the chewable lozenges was determined using digital pH meter (Milwaukee, USA), which was calibrated by standard solutions at pH 4.5 and pH 7. One gram of the weighed formulation was dispersed in 100 mL of distilled water and the pH was noted [5, 31].
Thickness
Thickness was measured using vernier caliper and expressed in mm [5,31].
In-vitro dissolution test
In principle, the dissolution test procedure employed for chewable pharmaceutical formulations should be the same as that for solid formulations. This concept is based on the possibility that a patient might swallow the dosage form without proper chewing and so the active compound will still need to be released in the gastro-intestinal tract to ensure the desired pharmacological action [32].
The release of polyphenols from lozenges was determined using USP dissolution testing apparatus type 2 (Electrolab, India) [33]. The dissolution test was performed at pH 6.8 for 30 min simulated the saliva medium and pH 1.2 for 1 hour simulated the gastric medium as shown in Table 2 [34].
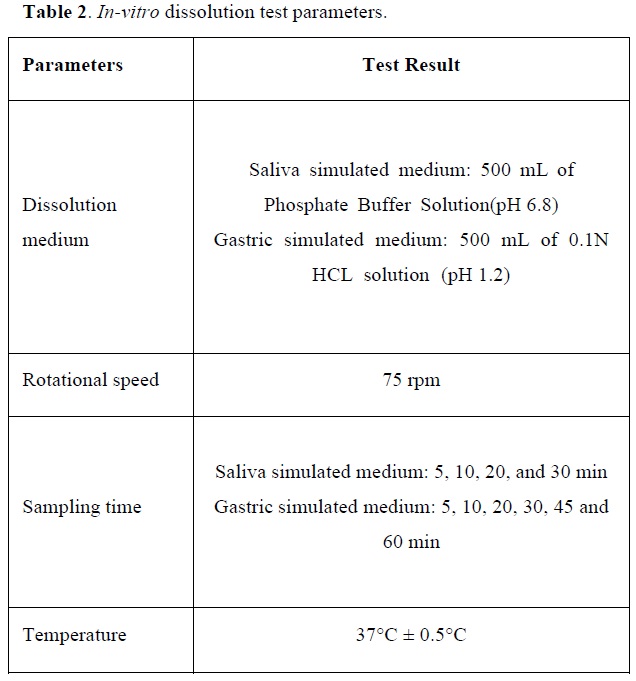
A sample (1 mL) of the solution was withdrawn from the dissolution apparatus at different time intervals and the samples were replaced with fresh dissolution medium. Folin-Ciocalteau assay was followed to quantify total polyphenols at 734 nm using a Shimadzu UV/Vis double-beam spectrophotometer as described by Zam [35].
Cumulative percentage release was calculated using the equation where A is the absorption of the withdrawn sample and A0 is the absorption of the extract at the same conditions.
Stability Studies
The stability study was conducted on the two formulas (F2 and F5). Chewable lozenges were tightly packed and stored at a cooling temperature (4°C) for three months, and the changes in the properties (appearance, stickiness and grittiness, pH, thickness and dissolution) was evaluated monthly [5,31].
Antibacterial efficacy
The antibacterial efficacy of the chewable lozenges on S. pyogenes and S. pneumonia was studied using agar-well diffusion method [36]. Bacterial strains were provided from throat swabs collections of the Microbiology Laboratory of Tishreen University Hospital, in Syria.
In this method, about 15-20 mL of Mueller-Hinton agar was poured on sterile Petri dishes and allowed to solidify. Agar surface of each plate was inoculated by spreading a volume of the bacterial suspension, which adjusted to match of a 0.5 McFarland standard (concentration 1.5 X 108 CFU/ml) using sterile cotton swab. Holes were formed on the surface of Agar plate, then 100 μL of each liquid chewable lozenges mixture was poured with micropipette in the hole. The plates were allowed to standby for 30 min and then incubated at 37°C for 24 h. The plates were then inspected for bacterial growth inhibition and the antibacterial activity was expressed as the diameter of inhibition zones produced by the liquid chewable lozenges mixture against test bacteria.
Statistical analysis
Descriptive statistics were used to summarize the study variables. Statistical analyses were conducted using the SPSS statistics 21 software. For comparison of means, ANOVA test was used and P values < 0.05 were considered significant.
RESULTS AND DISCUSSION
The shapes of the prepared chewable lozenges for each formulation were showed in Figure 1 and the physicochemical properties were summarized in Table 3.

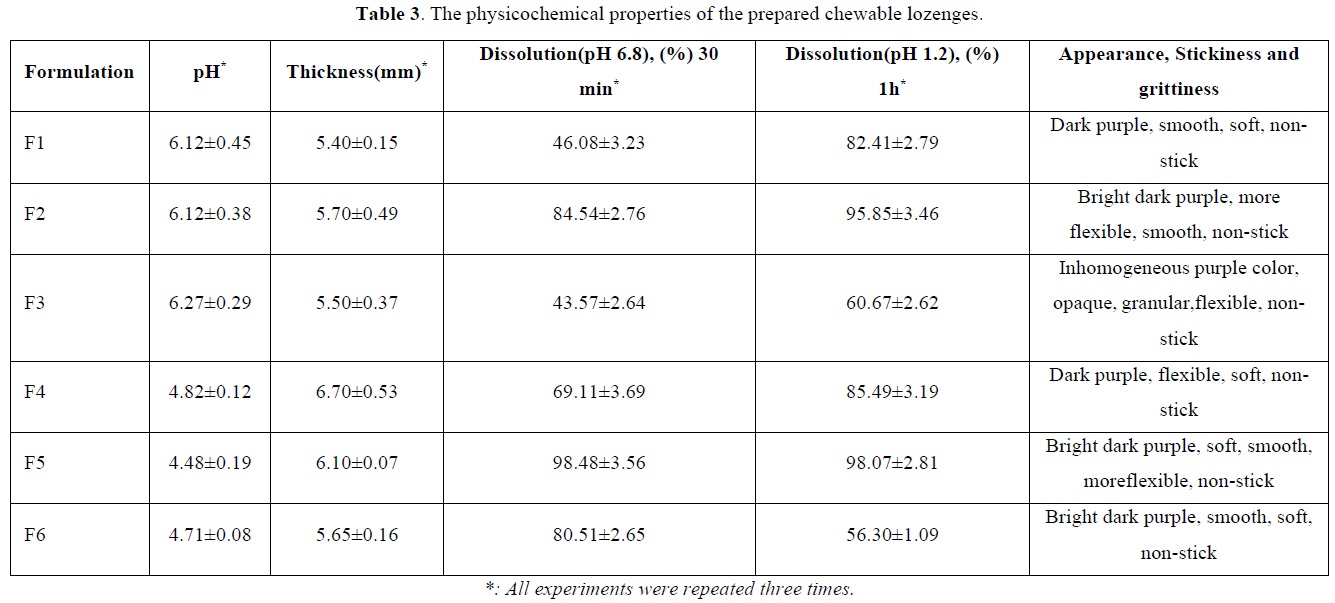
As noticed in Table 3, all the formulations were non-stick with different grades of purple color. The sugar formulations (F4, F5, and F6) had greater thickness and lower pH values compared with the sugar-free formulations (F1, F2, and F3) partially due to the addition of citric acid to the sugar formulations.
Generally, the sugar-containing formulations showed more release of polyphenols in both saliva simulated medium and gastric simulated medium, due to the presence of sugar, which is known to be water-soluble, making them disintegrating and dissolving rapidly. It has been previously proved that sugars such as sucrose and glucose as well as alcoholic sugars could be used in the preparation of fast-melting tablets because of their excellent physiological, chemical and technological properties [37]. The properties of swelling and the ability to form gel also play an important role in the behavior of the release of the active substance [38]. Hydrophilic functional groups (-OH, -COOH, and -NH2) in polymeric materials structure used in this study (gelatin, salep, and pectin) provide hydrogen bonding formation and thus high water absorption. This explains the positive effect of hydrophilic polymers on the swelling index [39]. As the gelatin swelling index also depends on ionization of these functional groups. Therefore, the medium pH, as well as electrolyte concentration and presence of other complexity factors, affect the ionization status of gelatin. It has been reported that the formulation containing gelatin and pectin had a low swelling index compared with ones containing gelatin alone [40], which is consistent with our results.
As noticed in Table 3 the release of polyphenols from formulations consisting of gelatin and salep only (F1, F2, F4 and F5) was negatively correlated with the amount of salep. This is consistent with previous results which proved that tablets containing the least concentration of salep had better dissolution and releasing of active substances [41]. This can be explained by the fact that increasing the concentration of solids in the formulation reduces porosity and water penetration, and thus slows the polyphenols release.
From the above results, the polyphenols release rate was highest in both mediums for the two formulations F2 and F5. Additionally, these two formulations showed more elasticity than other ones. Therefore, the stability study was conducted on these two formulations for three months and results are reported in Table 4.
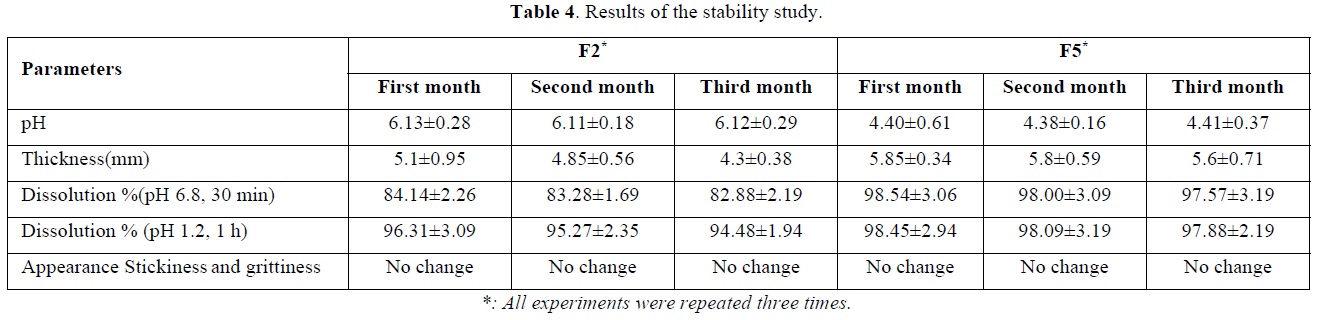
No significant changes (P<0.05) were observed in the prepared lozenges during the three months period at a cooling temperature (4°C). A slight decrease in the thickness of the formulation F5 was observed during storage and could be explained by the presence the sugar that binds the free water more strongly. The polyphenols release rate in both mediums also has not varied significantly due to the fact that the correlations between biopolymers (gelatin and salep), including the electrostatic interactions, hydrophobic interactions and hydrogen bonding, gives synergistic effects that lead to significant improvement in many mixtures [42]. Additionally, the cross-linking of proteins and polysaccharides play an important role in the functional characteristics of food systems [43]. In the same context, the correlation of polyphenols with biopolymers in the formulation may increase their physical stability, their antioxidant activity and their bioavailability [44], in which, it was reported in a previous study that the increase in the strength of gelatin gel could be attributed to the hydrogen bonds formed with polyphenols [45,46]. As well as that plant polyphenols have currently been explored as cross-linking agent for gelatin-polysaccharide crosslinking [47]. This cross-linking therefore stabilizes polyphenols and thus protects them from oxidation. Furthermore, the low storage temperature was an additional supplementary factor in maintaining the stability of polyphenols during a period of three months. It had been proved in previous studies that the storage at temperature (4°C) was better than (25°C) to maintain the stability and release of polyphenols from its different formulations [48].
The effect of the prepared chewable lozenges on two types of oral bacteria was shown in Table 5.
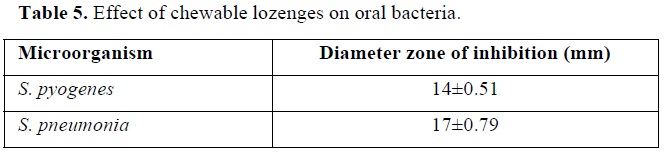
Results showed that the prepared chewable lozenges have a good effect against the two types of tested bacteria with a diameter zone of inhibition of 14 mm against S. pyogenes, and of 17 mm against S. pneumonia. This effect is due to the antimicrobial activity of myrtle berries, cinnamon and cloves extract, included in the prepared lozenges, which is attributable to their high content of phenolic components as earlier proved in our previous study [26]. It was previously proved that all oral isolates were sensitive to myrtle oil at 125-1,000 μg/mL by agar disk diffusion method producing inhibition zones of 8.1-41.25 mm in diameter and all of the S. pyogenes, S. mutans and C. albicans strains were sensitive to 62.5 μg/mL [49]. It had been also reported that both crude aqueous and methanolic extracts of cloves exert inhibitory effects on S. mutans [50]. Additionally, the ethanolic extract of cinnamon bark was found to significantly inhibit Streptococcus growth, and it was effective as mouthwash [51].
CONCLUSION
The present study focused on the formulation and evaluation of chewable lozenges supplemented with, myrtle berries, cinnamon and cloves extract. The use natural hydrophilic polymers, gelatin and salep, gave good results regarding the release polyphenols in both salivary and gastric media. Results also showed that the prepared chewable lozenges had a good activity against two types of oral bacteria S. pyogenes and S. pneumonia. Therefore, they could be suitable for the oral problems and could be used by children and elderly having a problem in swallowing. Lozenges were found to be stable when stored at (4ºC) for a period of three months.
REFERENCES
No Files Found
Share Your Publication :