-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Kazuo Sugimoto*, Takamichi Hattori, Yoshio Kitukawa, Akiyo Aotsuka, Takeshi Wada, Hitoshi Kubosawa and Shoichi Ito
Corresponding Author: Kazuo Sugimoto, Departments of Clinical Allergology, Neurology Clinic Tsudanuma, Funabashi, Japan.
Received: August 19, 2021 ; Revised: September 14, 2021 ; Accepted: September 17, 2021
Citation: Sugimoto K, Hattori T, Kitukaw Y, Aotsuka A, Wada T, et al. (2021) Appropriate Treatment using Povidone-Iodine for Atopic Dermatitis. J Clin Immunol Res Ther, 1(1): .
Copyrights: ©2021 Sugimoto K, Hattori T, Kitukaw Y, Aotsuka A, Wada T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
We have proposed a combination therapy using a disinfectant as a countermeasure against Staphylococcus aureus, which is often detected on the skin, in the treatment of moisturizer ointment and steroid ointment, which are common for the treatment of atopic dermatitis. During treatment, many cases experienced improvement in skin rash and dramatic improvement in laboratory data. The treatment method that combines this general treatment method with disinfection therapy was named Staphylococcal disinfection skin care method [1]. (In Japan, this treatment is called Isodine® therapy [2].)
By the way, Yamada et al. [3] reported a complication of colitis in patients with atopic dermatitis. Kira et al. [4] reported a complication of myelitis in patients with atopic dermatitis. Therefore, we also performed duodenal biopsy, neurology examination, and cervical spine MRI for many patients with atopic dermatitis. As a result, many cases of atopic dermatitis showed damage to the cervical spine and duodenum. In addition, we experienced many cases in which these disorders were observed at the same time in the same case [5,6,7]. Therefore, we estimated the involvement of the toxin (which is called superantigen [8,9]) produced by S. aureus not only in the skin but also in the intestinal tract and cervical spine, and concluded that atopic dermatitis is a superantigen disease [10]). Therefore, considering the importance of treatment against toxins that cause damage not only to the skin but also to multiple organs, we would like you to practice this treatment method that we have developed from the early stages of treatment for patients with atopic dermatitis.
CLINICAL BACKGROUND IN PATIENTS WITH ATOPIC DERMATITIS
1. Skin culture results
In the skin culture of 607 patients with mild to severe atopic dermatitis who first visited our hospital in 1995, methicillin-sensitive S. aureus was isolated in 55.0%, coagulase-negative staphylococci in 17.5%, both methicillin-sensitive S. aureus and coagulase-negative staphylococci in 17.5% and methicillin-resistant S. aureus in 1.6%. No growth was found in only 8.4% [1].
The frequency of MRSA examined in the same manner was 4.2% in 109 cases in 2003, which increased in a few years compared to 1.6% in 1995 [1-7].
2. Toxin produced by Staphylococcus aureus
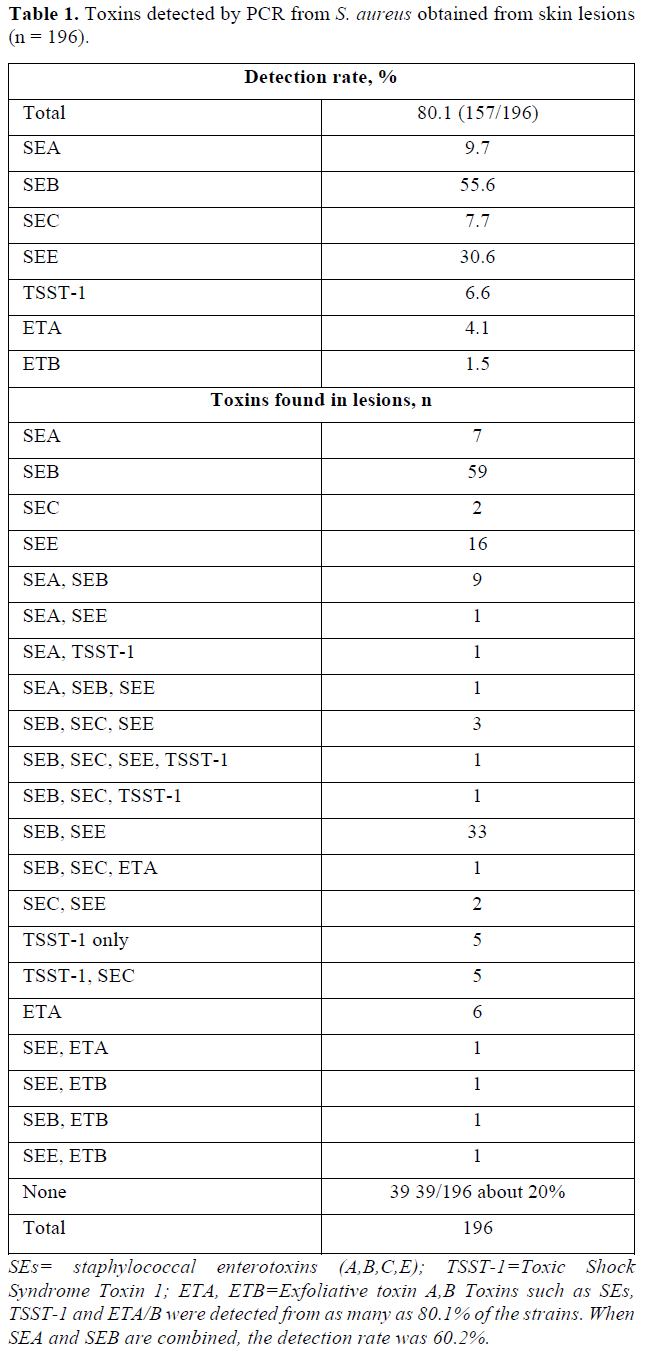
S. aureus produces many toxins. The toxin was measured using the PCR method, and the toxins included staphylococcal enterotoxin A (B, C, E), toxic shock syndrome toxin 1 and exfoliative toxin A (B).
The total detection rate of toxins was 80.1% from 196 S. aureus strains [7].
Currently, clinical antitoxin IgE antibody measurements are available for Staphylococcal Enterotoxin A (SEA) and SEB. The total rate for SEA and SEB detection was 60.2% [7].
As you can see in the Table 1, we have already reported the results of toxins produced by 196 strains of S. aureus.
The detection rates of staphylococcal enterotoxins A, B, C and E were 9.7, 55.6, 7.7 and 30.6%, respectively. The detection rate of toxic shock syndrome toxin 1 was 6.6% and those of exfoliative toxins A and B were 4.1 and 1.5% [7]. The positive rate of toxin was as high as 80.1% from 196 strains of S. aureus detected in atopic dermatitis [8-10].
3. Organ disorders other than skin disorders in atopic dermatitis
Regarding the neurological abnormalities [11] sometimes seen in patients with atopic dermatitis, we confirmed the neurological abnormalities in 89 out of 110 patients (80.9%) with atopic dermatitis. MRI was also used to observe cervical spine abnormalities in 54 of 69 atopic dermatitis patients (78.2%) who showed neurological abnormalities. Twenty-one of 32 atopic dermatitis patients (65.6%) were observed for both duodenal and cervical spine injuries [10] (Table 2).
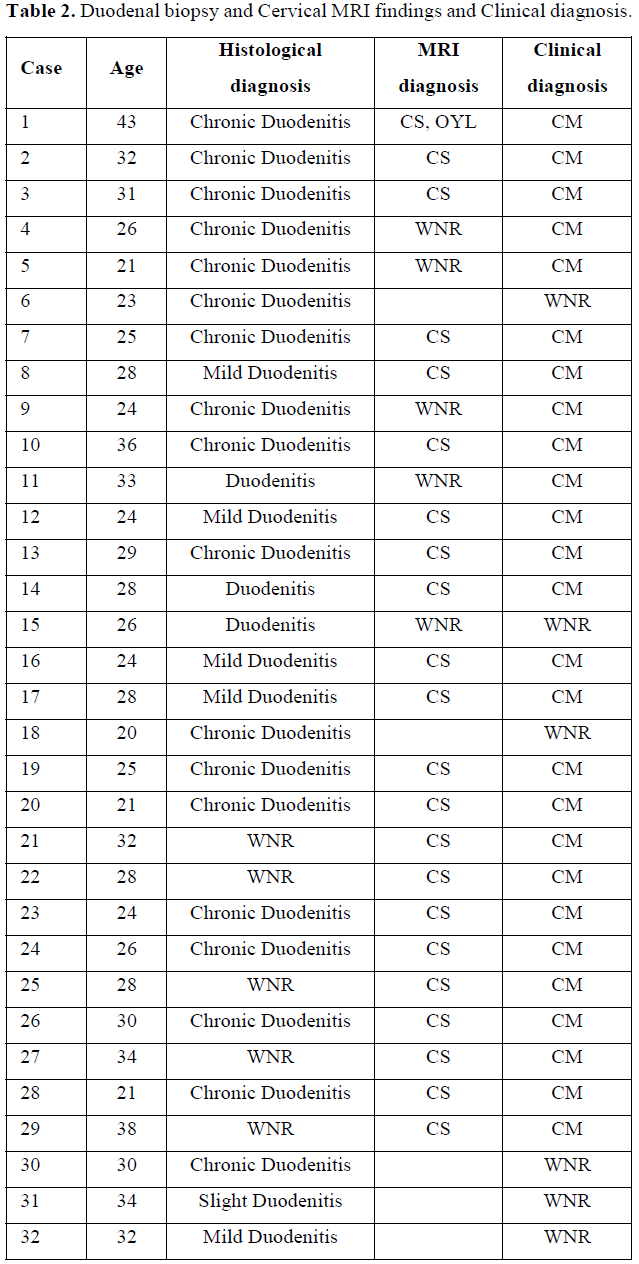
In addition, duodenal inflammation was observed in 43 of 53 patients with atopic dermatitis duodenal biopsy. Of the 12 patients who had repeated duodenal biopsies, 5 of the 12 patients who had repeated duodenal biopsies normalized duodenal inflammation and the rash with the staphylococcal disinfectant skin care method we developed for atopic dermatitis [10].
4. Clinical experience with this treatment
We have already reported in several papers that in many cases this treatment improves total IgE levels and antitoxin IgE antibody levels against Staphylococcal Enterotoxin A (SEA) and SEB [12,13] almost in parallel in a short period of time.
We have experienced a dramatic improvement in serum TARC levels in many cases with this treatment. We have already reported one case [14].
The sterilization time of Povidone iodine solution against S. aureus is said to be 20 to 30 sec. It is estimated that a considerable number of S. aureus remains in the 20 to 30 sec application period of Povidone iodine solution, as opposed to S. aureus in places such as the skin surface where organic substances (plasma components, etc.) are present. To support this, Haley CE [15] reported that a considerable number of MRSA bacteria remained even after applying povidone iodine solution to MRSA on the skin for 30 sec to 1 min.
Since the skin on the face is anatomically thin and vulnerable to irritation, we set the Povidone iodine solution on the face for 2 min as a countermeasure against S. aureus in patients with atopic dermatitis over 5 years old. And for other bodies, the application time of povidone iodine solution was set to 3 min, and a treatment method was proposed in which the povidone iodine solution was completely rinsed with water after application [1,16,17]. (We wrote in Paper 16 entitled Significance of Disinfectants for the Treatment of Atopic Dermatitis that the sterilization time for S. aureu povidone iodine solution is 5 seconds. But that was a mistake and we should have written that the sterilization time is 20-30 seconds. We apologize for correcting the mistake.)
It has already been reported that there is no problem with thyroid disorders by flushing the povidone iodine solution in a short time. For skin with atopic dermatitis under 5 years old, we use a diluted povidone iodine solution. And clinical improvement has been experienced in many cases with this application time. We also applied this treatment to 25 patients with atopic dermatitis in which MRSA was detected on the skin, and already reported that MRSA was able to be eradicated in all cases in a short period of time [2].
DISCUSSION
Atopic dermatitis is a disease whose main lesion is pruritus with repeated exacerbations and remissions, and it is defined that many patients have atopic predisposition. Many factors are involved in exacerbation and remission.
Kobayashi [18] reported that S. aureus is involved in the onset and exacerbation of atopic dermatitis.
We thought that the involvement of S. aureus is particularly important among the many factors involved in exacerbation and remission. We have developed Isodine® therapy [2], which is a combination of disinfectant therapy and general treatment, as a countermeasure against S. aureus in atopic dermatitis. We have experienced a short-term improvement in skin rash with this treatment in many cases. We also experienced dramatic improvements in laboratory test values in many cases.
The use of povidone iodine solution was described for the first time in atopic dermatitis in Japan’s Atopic Dermatitis Clinical Practice Guideline 2018 [19]. In this guideline, there is no medical basis to actively recommend the use of povidone iodine solution. It is difficult to treat with basic treatment such as topical steroids, and it may be considered as an adjunct therapy for cases in which infection is thought to be involved in the cause, but it should not be done easily because of safety concerns.
In addition, since this treatment improves the improvement of many test values of patients with atopic dermatitis in a relatively short period of time, we wondered if the disorder of atopic dermatitis could not be explained only by the skin. Therefore, after seeing reports that atopic dermatitis was a disorder of the intestinal tract and cervical spine, we tried duodenal biopsy and neurological diagnosis and examination. In many cases, not only skin but also multi-organ disorders were observed in the same case. It was presumed that the toxin produced by S. aureus detected on the skin of atopic dermatitis affects multi-organ damage [10,16,17].
In addition, although the frequency of MRSA detected on the skin of atopic dermatitis has increased recently, we have already been able to eradicate MRSA in all 25 cases of atopic dermatitis with this treatment method.
In Japan's 2019 Treatment Guidelines for MRSA Infections-Revised Edition [20], MRSA is detected in 10 to 30% of S. aureus detected in outpatients. This Isodine® therapy is effective as a countermeasure against MRSA, and it can be eradicated in all cases, so we would like to recommend it as an appropriate treatment for the treatment of atopic dermatitis.
By the way, already in 2003, Dhafer Laouini [21] showed that epicutaneous exposure skews the response to superantigen toward TH2 cells, leading to allergic skin inflammation and increased IgE synthesis, which are characteristic of atopic dermatitis. Furthermore, they suggest that therapies that effectively decrease skin colonization with superantigen-producing organisms might have a beneficial effect in atopic dermatitis.
Therefore, we sincerely hope that the treatment method we have developed will spread from the early stage of treatment for patients with atopic dermatitis as a countermeasure against toxins that cause damage not only to the skin but also to multiple organs.
REFERENCES
No Files Found
Share Your Publication :