-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Ateksha Khanna*
Corresponding Author: Ateksha Khanna, Medanta Hospital Gurgaon, D-88, Anand Niketan, New Delhi-110 021 India
Received: January 15, 2021 ; Revised: February 16, 2021 ; Accepted: February 18, 2021
Citation: Khanna A. (2021) Epitome of Clinical Evidence Systematic Reviews in Endodontics. J Infect Dis Epidemiol Res, 1(1): 1-7.
Copyrights: ©2021 Khanna A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Systematic reviews and meta-analysis are the paramount level of evidence, based on which clinical guidelines are formatted. Furthermore, they guide clinicians to provide optimal evidence-based care to their patients (Gopalakrishnan and Ganesh Kumar, 2013).
Systematic Reviews are constructed to identify, appraise and summarize all the available evidence in accordance with a pre-determined criterion to answer a focused question.
Systematic reviews form the highest level of evidence (Phillips et al 2008, Evans 2003, Fleisher et al 2005 and Guyatt et al 2000) and are imperative to the dynamic changes in medicine. An understanding and implementation of systematic reviews is mandatory for all healthcare professionals.
It is important that abstracts of a Systematic Review should ideally adhere to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
According to the Cochrane Collaboration the researchers must follow a strict and explicit methodology in order to minimize bias and produce reliable findings that can be used in decision making. The standard of systematic reviews and meta-analysis published from 2009-2016 has been described as “medium” thereby affecting the Evidence based practice in Endodontics (Kattan et al 2018).
The aim of this editorial is to direct authors writing reviews to produce manuscripts with minimal issues and the ease of understanding by the reader.
WHAT IS THE AIM OF A SYSTEMATIC REVIEW?
Depending on the nature of the data, the results of a systematic review can be summarized in text or graphic form. In graphic form, it is common for different trials to be depicted in a plot where the point estimate and 95% confidence interval for each study are presented on an individual line. When results are mathematically combined (a process sometimes referred to as pooling), this is referred to as meta-analysis. Graphically, the pooled result is often presented as a diamond at the bottom of the plot [1-6].
SYSTEMATIC REVIEW METHODOLOGY
A good systematic review is the one which has a clear methodology. The methodology involves the following steps.
Formatting a focused question
There should be clear instructions on the question to be answered with inclusion and exclusion criteria. Framework by [11] Hassing 1999 in formatting a question (PICO/PECO) is as follows.
Patient Population
E/I Exposure/Intervention Comparison:
Outcome: Modifications are added if any changes are made to the protocol such as defining populations, outcomes, interventions or study designs.
Inclusion and Exclusion Criteria: Exclusion and inclusion criteria should be determined related to the question cautious of the introduction of any bias.
THE SEARCH BEGINS
Commonly used are Medline/PubMed, Web of Science, Embase, Metapress, Scirus, Biosis, eTblast, Cochrane databases of randomized trials or systematic reviews, Google Scholar, DARE (Database of Abstracts of Reviews of Effectiveness), HTA (Health Technology Assessment Database, LILACS (language specific database) and SIGLE (System for information on grey literature in European Achieve).
Search should be exhaustive using multiple resources online and printed without language barriers.
QUALITY ASSESSMENT
Quality assessment and minimizing bias is an integral part of the systematic review. Some points to be kept in mind are acknowledging all the evidence and seeking expert opinion.
These quality assessments are used for analyzing heterogeneity and decisions regarding suitability of meta-analysis. They will also aid in assessment of interference and future recommendations.
EVIDENCE SUMMARY
Synthesis of the data consists of tabulation of the characteristics of the study, quality and the use of statistics to explore differences between various studies and their combined effects.
Synthesis and summarizing of the evidence should be done by reviewers’ independently [12]. Advance planning should be implemented for determining heterogeneity. Failure to format a meta-analysis should be promptly replaced by a sub-meta-analysis.
RESULTS OF SYSTEMATIC REVIEW
Publication bias should be explored. Heterogeneity should be explored to determine if the summary can be trusted, if not, the effects generated in high-quality studies should be utilized for generating inferences.
SYSTEMATIC REVIEWS IN ENDODONTICS
The published data in dentistry is increasing exponentially. However, it is difficult for the clinician to ascertain if the information is accurate and valuable. Systematic reviews are a remedy to this problem as they are ranked as the highest level of evidence. Unfortunately, emphasis is currently being placed on reviewing the quality of these reviews and making dentists’ aware of the shortcomings of some of these reviews.
LIMITATIONS OF SYSTEMATIC REVIEWS
Some authors have questioned the quality of systematic studies in endodontics. Spanberg [13] stated that good endodontic literature is rare and will take some time to improve. To support this statement, he included three studies which failed in their objectives and were doubted as being a part of the literature. In response to this letter, Sathorn blamed the editors and reviews for publishing these studies.
An assessment into the methodology of systematic reviews in dentistry carried out by Glenny [14] highlighted that a substantial number of the reviews were substandard and the conclusions may be misguiding to dental professionals. The author reviewed 65 systematic reviews between 1990-2001 and found serious shortcomings in the search criteria, quality assessment of studies, no examination of heterogeneity and inappropriate pooling of data.
A review of the quality of 16 meta-analysis reports in endodontology published between 2001-2009 showed that the published meta-analysis had high AMSTAR scores [quality scoring system for systematic reviews] in most of the areas but weaker areas included failure to disclose the conduct of assessment of publication bias. An editorial: Is it time to recycle “garbage in-garbage out” [15] systematic reviews pointed the shortcomings in the above review especially with the inappropriate use of AMSTAR scoring system (as discussed below).
Endodontics are working hard to publish systematic reviews and meta-analysis which is useful for the practitioners. But is this a global trend towards good quality research?
This has highlighted the importance of availability of rigorous quality assessment tools for assessment of systematic reviews to ensure that dental care professionals are provided with highest level of evidence regarding an intervention which would enable them to make essential decisions regarding patient care.
QUESTIONS TO BE ASKED WHEN INTERPRETING AS SYSTEMATIC REVIEW [12].
QUALITY OF SYSTEMATIC REVIEWS
Mohar [16] defined quality as a plausibility that the design of a systematic review will bring about unbiased results.
Quality assessment of systematic reviews ranges from a simple checklist which was developed in 1990s to a complex and cumbersome checklist known as Overview Quality Assessment Questionnaire (OQAQ).
This was followed by the release of Quality of Reporting of Meta-analyses (QUOROM) statement in 1996 by Moher et al and the implementation of QUOROM tool which is a combination of a flow chart and checklist to assess the. quality of a meta-analysis.
QUOROM tools saw an improvement in the quality of systematic reviews and meta-analysis. The release of Consolidated Standards of Reporting Trials (CONSORT) statement by Begg [17] saw a similar improvement in the quality of studies being conducted which empowered the clinician with best available evidence. The increased attention of the journal editors and reviewers to the general methodological quality of reports also played a role. The AMSTAR tool is currently the most widely used tool in assessing the quality of methodology of systematic reviews.
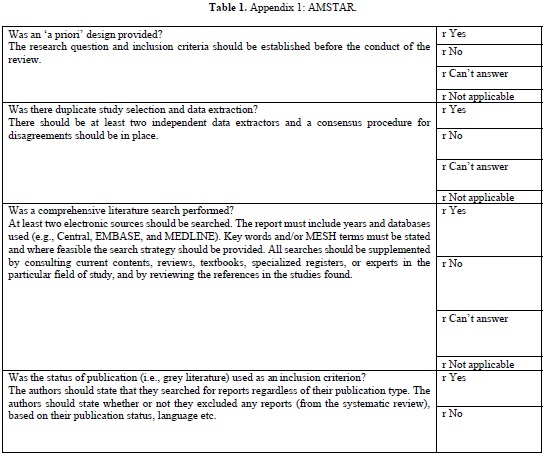
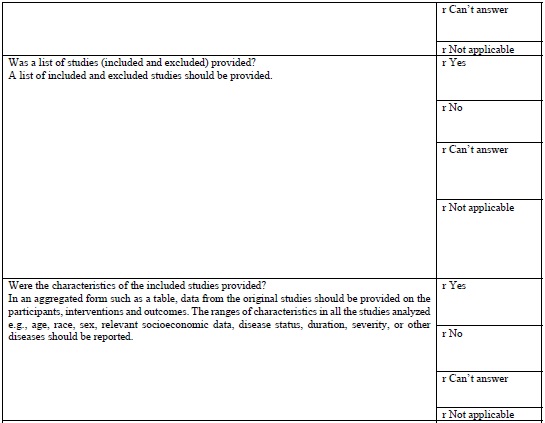
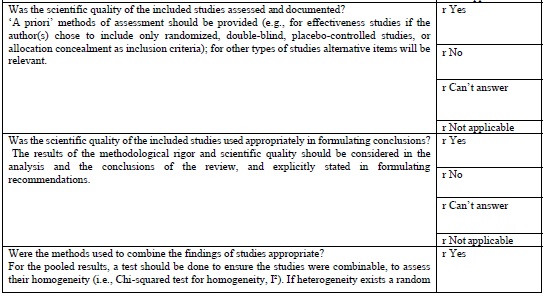
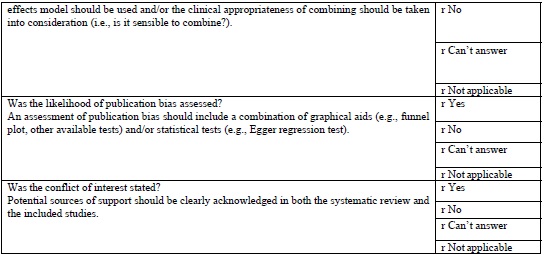
AMSTAR TOOL
The AMSTAR tool (Table 1 (Appendix 1)) which is currently the most widely used tool in assessing the quality of methodology of systematic was developed in 2007. This is a 11-item questionnaire each item is given a score of 1 if the criteria are met and score of 0 if criteria is not met, is unclear, or is not applicable.
AMSTAR quality assessments are divided into three ranges
It provides a summary score, which is helpful for clinicians making decisions [18].
According to Shea [19], careful psychometric assessments determined that AMSTAR has good face and content validity for measuring the methodological quality of systematic reviews and their clinical relevance.
As of this date, AMSTAR has been used by professional health care associations and other policy institutions, and has gained in respectability, reliability, reproducibility. However, AMSTAR has only been tested on randomized control trials evaluating treatment interventions its use is limited to evaluate diagnostic, prognostic studies.
There have been concerns regarding inappropriate use of AMSTAR in evaluating systematic reviews in Endodontology. In a review of meta-analysis using AMSTAR Suebnukarn 2010 revealed that the meta-analysis of various studies in endodontics had high AMSTAR scores. He was criticized for the inappropriate use of AMSTAR, in observational studies, non-randomized controlled trials [20].
CONCLUSION
In the current flood of publications, a great volume of systematic reviews and meta- analysis are available. Systematic reviews have become prominent in endodontics due to the rapidly growing emphasis on evidence-based medicine [21-23].
Ironically not all of the systematic reviews are of high quality. Hence readers must develop a critical attitude to interpret various publications.
Furthermore, clinicians performing systematic reviews and meta-analysis should stay focused to the question, have a vigorous protocol in place, thoroughly investigate clinical and statistical heterogeneity with attempts to minimize bias [24-27].
Journal reviewers and editors shouldn’t overlook poorly conducted reviews.
The development of AMSTAR tool has provided us with the ability to assess the methodological quality of the systematic review which is an essential determinant in the overall quality of a systematic review. This has helped the clinician to use evidence-based medicine in everyday practice [20].
Systematic reviews are powerful toots that influence clinical arena. On the contrary if improperly performed can be misleading.
With the increasing awareness of systematic reviews is this an awakening of a new trend towards good quality endodontics?
No Files Found
Share Your Publication :